BillionToOne Publishes UNITY Fetal RhD and Fetal Antigen NIPT Clinical Validity Demonstrating >99.9% Accuracy
Health & Medicine - The Offspring Session originally published at Health & Medicine - The Offspring Session
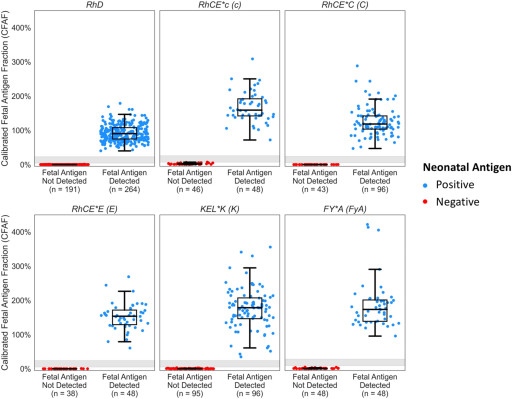 In a clinical validation study published on Scientific Reports, UNITY RhD NIPT and Fetal antigen NIPT demonstrated 100% sensitivity, 100% specificity, 99.9% precision with 0.1% no-call rate across 3,921 NIPT assays.
In a clinical validation study published on Scientific Reports, UNITY RhD NIPT and Fetal antigen NIPT demonstrated 100% sensitivity, 100% specificity, 99.9% precision with 0.1% no-call rate across 3,921 NIPT assays.
MENLO PARK, Calif., August 11, 2023 (Newswire.com) - BillionToOne, experts in fetal DNA quantification, announced today that Scientific Reports published a study1 of the UNITY Fetal Antigen Noninvasive Prenatal Test (NIPT). The validation study marks an inflection point for patients who are at risk of Hemolytic Disease of the Fetus and Newborn (HDFN) due to the assay's unmatched accuracy coupled with its novel approach.
In this validation study co-authored by scientists at BillionToOne and Brigham and Women's Hospital and senior-authored by Dr. Kathryn Gray, MD, PhD, the UNITY Fetal Antigen NIPT correctly determined the antigen status for 1,061 preclinical samples resulting in 100% sensitivity and specificity and achieved 99.9% precision on 1,683 clinical samples. Clinical samples with known outcomes (neonatal antigen genotype/serology) were also evaluated with 100% concordance across 93 antigen evaluations in 30 alloimmunized pregnancies. Impressively, 100% accuracy was achieved while maintaining a low no-call rate of 0.1%.
HDFN is a serious condition that occurs when a pregnant patient's antibodies cross the placenta and attack the fetal red blood cells. When most severe, HDFN can cause life-threatening anemia in the fetus. Prior to the availability of UNITY Fetal Antigen NIPT, identifying patients at highest risk of HDFN was error-prone and cumbersome, necessitating a partner's sample and invasive diagnostic testing.
This new approach with UNITY Fetal Antigen NIPT is set to revolutionize identification and management for patients at risk of severe HDFN due to the assay's greater accuracy and convenience compared to existing tests like amniocentesis, which comes with many risks, particularly for patients at risk of HDFN.
The publication also highlights how UNITY Fetal Antigen NIPT can also be used to nearly eliminate unnecessary prophylactic treatment for 40% of RhD-negative pregnancies. The assay's unique capability to accurately assess non-deletion RHD gene variants, which are more prevalent in non-European populations, underscores the test's applicability and validity across ethnically diverse populations. This capability reduces the assay no-call rate to 0.1%, significantly lower than any previous RhD assays and addresses any potential concerns against front-line RhD NIPT screening.
"At BillionToOne, we are uniquely positioned to do the unthinkable with genetic testing due to our QCT technology," said Oguzhan Atay, PhD, CEO of BillionToOne. "The published validation data for UNITY Fetal Antigen NIPT is a perfect example of this, ultimately setting a new standard for patients and providers in the management of HDFN." As stated in the publication, "the clinical implementation of NIPT can significantly improve the standard of care for both prevention of RhD alloimmunization and management of alloimmunization in pregnancy."
About the UNITY Fetal Antigen NIPT
The UNITY Fetal Antigen Test Screen was first launched in September 2022 for pregnant patients alloimmunized against RhD, C, c, E, K (Kell), and Fya (Duffy) red blood cell (RBC) antigens, and can be performed as early as 10 weeks of gestation. Using BillionToOne's proprietary molecular counting technology or Quantitative Counting TemplatesTM (QCTs), this NGS-based assay detects the presence or absence of the genetic variants that code for corresponding fetal antigens to accurately identify which patients are truly at highest risk for developing HDFN. Since its launch, over 1,000 UNITY Fetal Antigen NIPT have been ordered for clinical use. About 60% of those patients received a "Not Detected" Fetal Antigen result, sparing the expecting parents from unnecessary follow-ups and undue anxiety.
About UNITY Fetal RhD NIPT
The UNITY RhD NIPT was launched in 2020 for pregnant patients with RhD-negative blood type at the gestational age of 10 weeks or higher. Up to 40% of RhD-negative pregnant individuals carry an RhD-negative fetus and do not need the administration of Rho(D) immune globulin. The QCT technology-based quantitative approach with NGS enables the assay to detect fetal RhD status both for the common RHD gene deletion and other RHD variants such as RHDΨ, significantly reducing the no-call rate while maintaining 100% sensitivity and specificity.
About BillionToOne
Headquartered in Menlo Park, California, BillionToOne is a precision diagnostics company on a mission to make molecular diagnostics more accurate, efficient, and accessible for everyone. The company's patented QCT molecular counter platform is the only multiplex technology that can accurately count DNA molecules at the single-molecule level.
molecular counter platform is the only multiplex technology that can accurately count DNA molecules at the single-molecule level.
For more information, please visit www.billiontoone.com.
For more information about the trial or the collaboration, please contact [email protected].
1 Alford, B., Landry, B.P., Hou, S. et al. Validation of a non-invasive prenatal test for fetal RhD, C, c, E, K and Fya antigens. Sci Rep 13, 12786 (2023). https://doi.org/10.1038/s41598-023-39283-3
Contact Information:Courtnay Davis
Events Marketing Manager
[email protected]
6504602551
Original Source: BillionToOne Publishes UNITY Fetal RhD and Fetal Antigen NIPT Clinical Validity Demonstrating >99.9% Accuracy
The post BillionToOne Publishes UNITY Fetal RhD and Fetal Antigen NIPT Clinical Validity Demonstrating >99.9% Accuracy first appeared on The Offspring Session.
Health & Medicine - The Offspring Session originally published at Health & Medicine - The Offspring Session